Commentary
Of all the antiviral drugs for COVID-19, Pfizer’s Paxlovid has been the most successful. Not for its safety and efficacy, but for its ability to earn the company billions in profits despite being largely ineffective for most people.
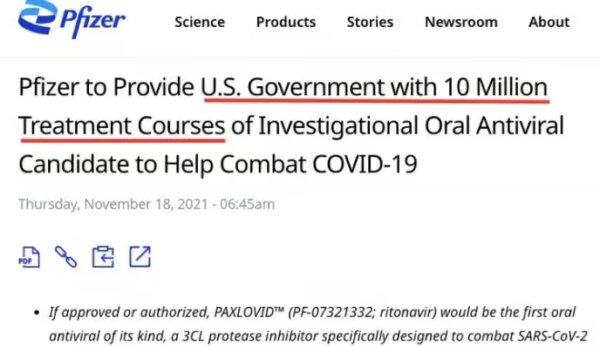
In November 2021, before any data had emerged, the Biden administration committed to
purchasing 10 million treatment courses of Paxlovid worth $5.3 billion, pending authorization by the U.S. drug regulator.
One month later, Paxlovid was
granted emergency use authorization by the Food and Drug Administration (FDA) for use in adult and pediatric populations ages 12 years or older.
The authorization was based on
early trial data showing the drug could reduce hospitalizations or death (89 percent relative risk reduction, 6 percent absolute risk reduction) in high-risk patients who were unvaccinated and had no prior exposure to COVID-19.
But the problem was that most Americans by that time (December 2021) had already been
vaccinated against COVID-19 or had prior
exposure to the virus, making the trial results irrelevant to the majority of people.
Pfizer had to prove that its drug could benefit a broader market.
The manufacturer commenced the
EPIC-SR trial, investigating the use of Paxlovid in unvaccinated people and vaccinated people with at least one risk factor for COVID-19.
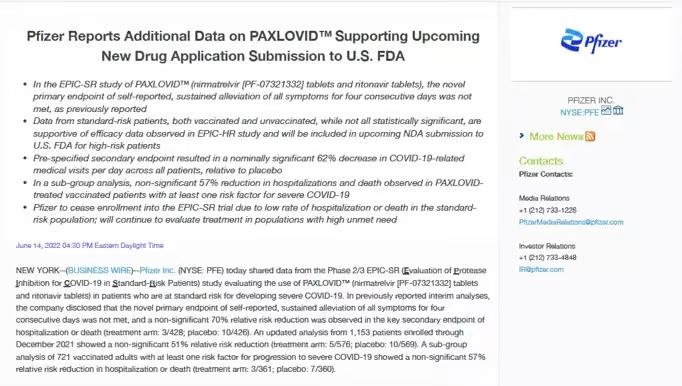
By July 2022, however, Pfizer stopped enrolling participants “due to a very low rate of hospitalization or death observed in the standard-risk patient population.”
In a statement, the company
announced that Paxlovid had failed to meet its “novel primary endpoint of self-reported, sustained alleviation of all symptoms for four consecutive days.”
In other words, Paxlovid—a combination of nirmatrelvir and ritonavir—made no significant difference in alleviating symptoms of COVID-19 compared with a placebo among nonhospitalized patients.
Pfizer stated that it was difficult to find a benefit in a population that was already at a low rate of hospitalization or death from COVID-19.
One year later, in August 2023, Pfizer quietly published the unfavorable findings on
ClinicalTrials.gov, without any fanfare or media attention. In fact, the media continued to
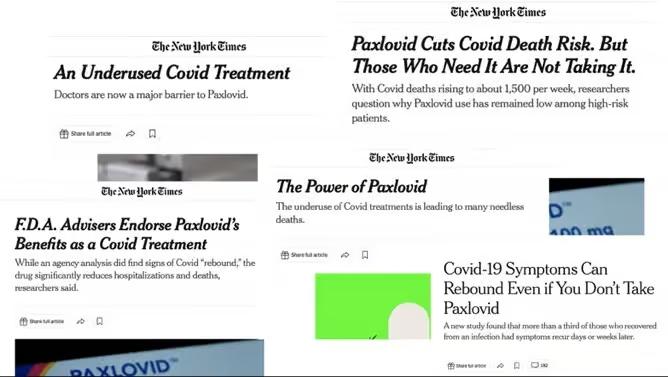
promote the benefits of Paxlovid to the wider public.
The New York Times, for example, ran multiple stories during the pandemic about the “Power of Paxlovid,” encouraging more people to take the drug and criticizing its underuse.
Simultaneously, Pfizer stoked public fear by overinflating the risk of COVID-19, paving the way for doctors to prescribe drugs such as Paxlovid to manage the disease. Sometimes, the claims were misleading.
Pfizer, for example, posted on social media platform X that three out of four U.S. adults were at “high risk” for severe COVID-19, but then cited a study in the advertisement that did not support the claim—so far, the misleading post has been viewed 11.6 million times.
“This is ridiculous,” Dr. Walid Gellad, professor of medicine at the University of Pittsburgh, posted. “I don’t know how it is legal ... 3 out of 4 adults are not at high risk of severe Covid.”
That didn’t stop FDA Commissioner Dr. Robert Califf from taking to social media to promote the benefits of Paxlovid.
He
posted that the drug could reduce the risk of developing “long COVID” based on weak evidence, and
admitted to “cheerleading” the use of Paxlovid because he felt overall “the evidence was strong.”
Dr. Califf copped criticism for his lack of impartiality as the head of the regulator but justified his actions in a “public health emergency.”
Regulatory affairs expert Jessica Adams said it was a poor excuse.
“Something is really wrong with public health ‘leadership’ if it thinks that every norm can be thrown out the window in an emergency,” Ms. Adams said. “The FDA has learned nothing during the pandemic and is setting terrible precedents for future emergencies.”
By 2023, reports of people experiencing “rebound” symptoms after using Paxlovid were increasing. Authorities could no longer
claim that it was “rare.”
High-profile officials such as former CDC Director Dr.
Rochelle Walensky, former National Institute of Allergy and Infectious Diseases Director Dr.
Anthony Fauci, President
Joe Biden, and First Lady
Jill Biden all had reported a rebound of COVID-19 symptoms after completing a course of Paxlovid.
Dr. Califf
dismissed concerns about rebound, saying it was all just a “distraction,” but a study
published in JAMA Network showed that symptomatic rebound in people with mild to moderate COVID-19 was as high as 25 percent after taking Paxlovid.
In May 2023, the FDA granted Paxlovid
full approval for managing mild to moderate COVID-19 infections in adults at high risk of developing severe disease (including vaccinated adults, despite no data showing benefit in this population).
In early April, Paxlovid was back in the spotlight after the EPIC-SR trial was finally
published in the New England Journal of Medicine, almost two years after Pfizer
announced the futility of the study in July 2022.
Regardless of all the positive media coverage and promotion of Paxlovid by public health officials and government advisers, the evidence is clear.
Paxlovid, which now
costs $1,400 for a five-day course, has shown benefit only in a very rare population—that is, unvaccinated people who’ve never encountered the virus and are at high risk of serious COVID-19.
Views expressed in this article are opinions of the author and do not necessarily reflect the views of The Epoch Times.